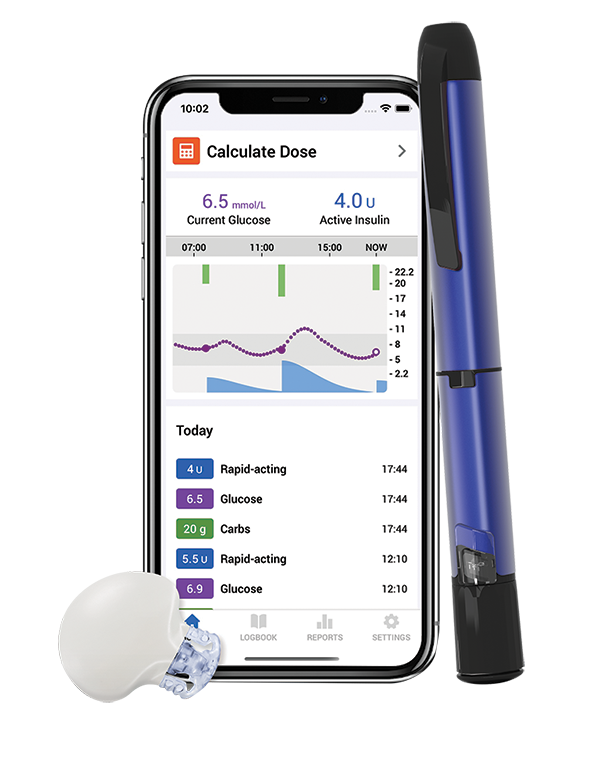
What is a Smart MDI System^?
The Smart MDI system combines the power of our latest innovation in continuous glucose monitoring (CGM), Guardian™ 4 Smart* CGM system with the InPen™ Smart** Insulin Pen System.
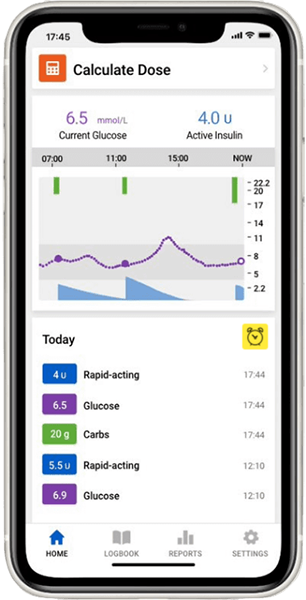
The InPen™ system is designed to assist with tracking of insulin doses and dose calculations.
Guardian™ 4 CGM system tracks your glucose levels 24/7 every 5 minutes, whilst the InPen™ system helps you take the right insulin dose at the right time.
^ Components sold separately.
Note1: A healthcare professional should assist in programming of the device prior to use, based on various patient-specific criteria and targets
Note 2: For additional product and safety information, please consult the Instructions for Use.
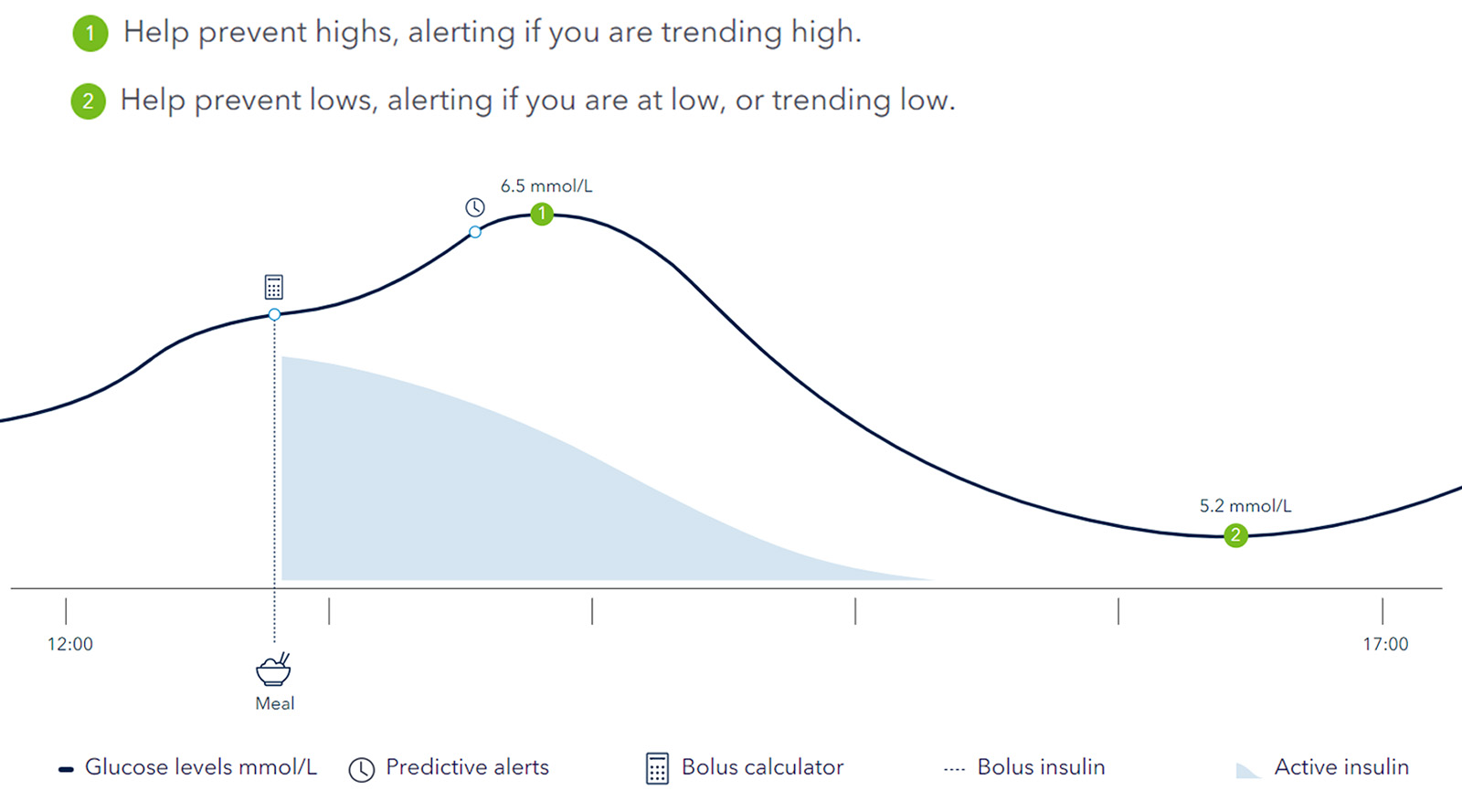
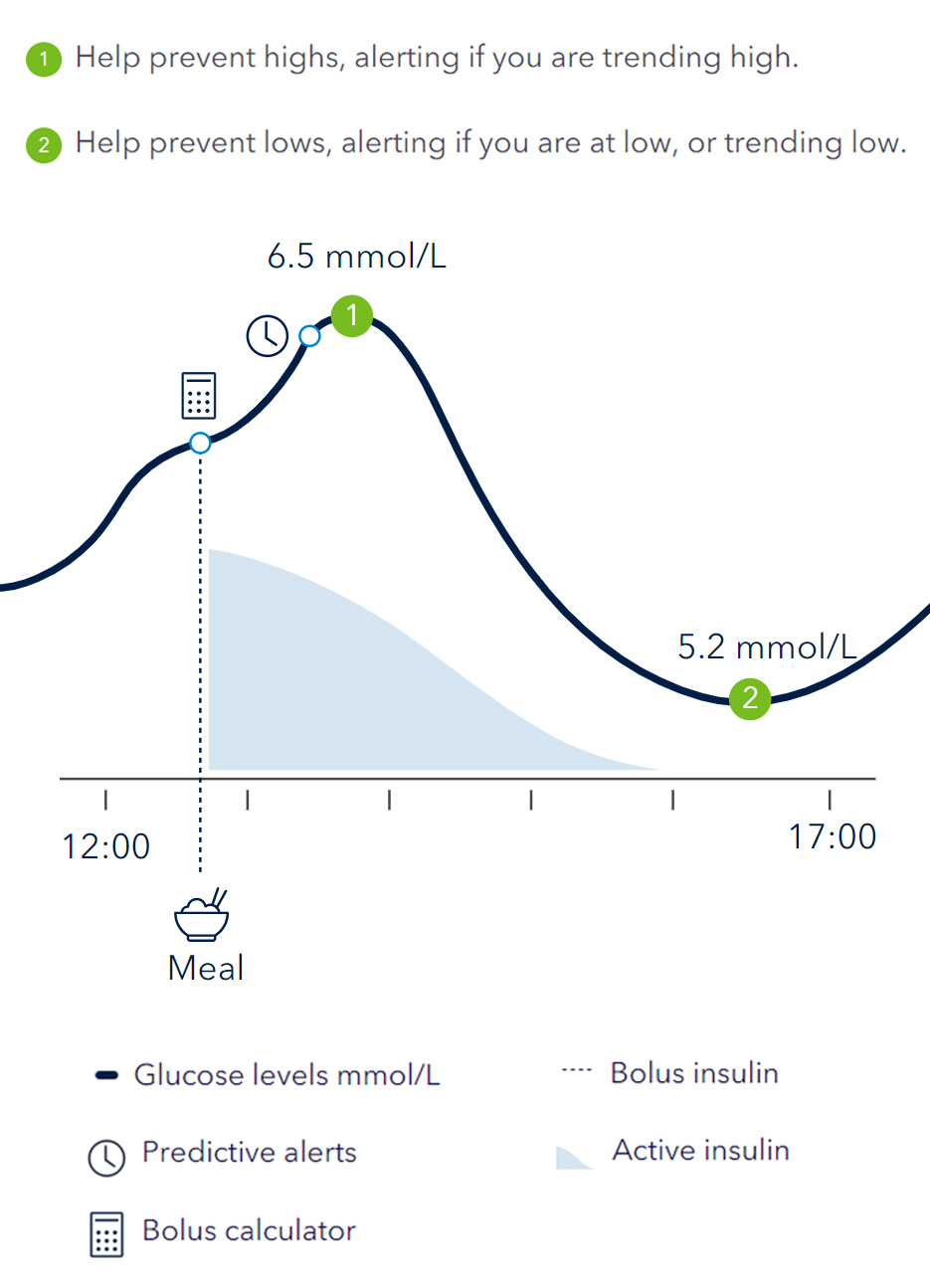
Combining the power of real time glucose readings with predictive alerts and personalised insulin dosing* to help you stay ahead of your glucose levels1,2. The Smart MDI systems can help to improve your Time in Range3(TIR).

InPen™ Smart** insulin pen system
The InPen™ system tracks active insulin, logs dose activity and can help your patients to take the right dose at the right time†.

Guardian™ 4 system
Guardian™ 4 system allows you to stay ahead with predictive alerts up to 60 minutes before a high or low event. Sensors can be worn for up to 7 days and record your glucose levels automatically every 5 minutes, 24/7 with no calibrations†.
Smart MDI system^
Note2: For additional product and safety information, please consult the Instructions for Use.
† Regarding Zero Calibrations, if sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide.
Know before you go low or high with real-time blood glucose readings, trends and alerts.
Ready to buy?
Since Smart MDI is Guardian System 4 plus InPen devices, InPen must be purchased from Medtronic or our eShop for ONLY $195 (RRP $595).
Guardian System 4 can be purchased through Medtronic or the NDSS (if eligible).
Frequently asked questions
The Smart MDI System brings together an ecosystem of smart tools that provide real-time insights and comprehensive reports to make it easier to manage life on MDI.
- The Guardian™ 4 system is a real-time, Continuous Glucose Monitoring (CGM) system indicated for the management of diabetes in persons aged 7 years and older.
- The InPen™ system is intended for single-patient use by people with diabetes for the self-injection of a desired dose of insulin. For patients who do not self-manage their diabetes, the use of InPen™ should always be under the supervision of a caregiver.
We’ve updated our standalone CGM system app and hardware to improve the customer experience. These are the most significant updates that can be found in the Guardian™ 4 system:
- The previous system was called the Guardian™ Connect system. This next generation of our Smart CGM is called the Guardian™ 4 Smart* CGM System
- The Guardian™ 4 system now requires zero calibrations†
- Non-adjunctive labeling. This means a user can now make treatment decisions based on the glucose value shown by the system, without the need for a confirmatory finger prick.
*Smart CGM predicts future high and low sensor glucose events up to 1 hour in advance and provides access to additional algorithms and insights that can inform users of clinically relevant glucose patterns. Please refer to IFU.
† If sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide.
At this time, InPen™ system is compatible with Novo Nordisk NovoRapid®, Novo Nordisk Fiasp® and Novo Nordisk Insulin Aspart Injection, 3mL cartridges (300 units).
Colour availability varies by region. Currently, the following colour is available:
- Blue InPen™ compatible with Novo Nordisk insulin cartridges
The Guardian™ 4 system and InPen™ system apps have a wide range of compatibility with several Apple® and Android™ devices. You can find updated compatibility information here on our website. We recommend checking compatibility and switching off automatic operating system updates to ensure the best possible user experience.
InPen™ system is the first available Smart** insulin pen that uses Bluetooth® technology and intelligence through an easy-to-use Smartphone app to help people administer correction and meal-time insulin doses.
**Smart insulin pen connects to a mobile app to provide dosing calculations, reminders and CGM system integration. Please refer to IFU.
Your Healthcare Professional or Medtronic representative may provide training on the Smart MDI system. Additional resources can be found at the Medtronic Diabetes My Learning page.
Yes, multiple InPen™ Smart insulin pens can be synced with one app account.
Cartridges are not included with InPen™ system. Your Healthcare Professional will provide you with your prescription for your diabetes management supplies.
References
1. Abraham SB, et al. Improved Real-World Glycemic Control With Continuous Glucose Monitoring System Predictive Alerts. Journal of Diabetes Science and Technology 2021; 15(1):91–97
2. Smith M, et al, E5 , SIPs Improve Time Below Range in MDI Therapy, AMCP Congress 2020
3. Adolfsson P, Væver Hartvig, Kaas A, Møller JB, Hellman J. Increased Time in Range and Fewer Missed Bolus Injections After Introduction of a Smart Connected Insulin Pen. Diabetes Technology & Therapeutics, 2020;22(7):online ahead of print
4. Dicimbrini I et al, Effects of real-time continuous glucose monitoring in type 1 diabetes : a meta-analysis of randomized controlled trials, Acta Diabetologica 2020 doi: 10.1007/s00592-020-01589-3
^ Components sold separately.
† if sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide.
* Smart CGM predicts future high and low sensor glucose events up to 1 hour in advance and provides access to additional algorithms and insights that can inform users of clinically relevant glucose patterns. Please refer to IFU.
** Smart insulin pen connects to a mobile app to provide dosing calculations, reminders and CGM system integration. Please refer to IFU.
ALWAYS FOLLOW THE DIRECTIONS FOR USE.
For detailed information regarding indications, contraindications, warnings, precautions, and potential adverse effects, please consult the information for use at: www.medtronicdiabetes.com.au/
InPen™ is intended for single-patient use by people with diabetes for the self-injection of a desired dose of insulin. The Guardian™ 4 Sensor is intended for insertion into persons ages 7 years and older.
Regarding Zero Calibrations, if sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG) readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
This patients testimonial relates an account of an individual's response to the treatment. The account is genuine, typical and documented. However, the individual's response does not provide any indication, guide, warranty or guarantee as to the response other persons may have to the treatment. The response other persons have to the treatment could be different. Responses to the treatment discussed can and do vary and are specific to the individual patient. Please consult your healthcare professional for a full list of benefits, indications, precautions, clinical results and other important medical information that pertains to the therapy or products discussed.
©2023 Medtronic Australasia Pty Ltd. All rights reserved. 2 Alma Road, Macquarie Park, NSW 2113. SpringCM Approval# 13087-082023