

ACCESSPLUS+ PUMP PROGRAM
Do you want to try the latest & greatest in diabetes tech now?
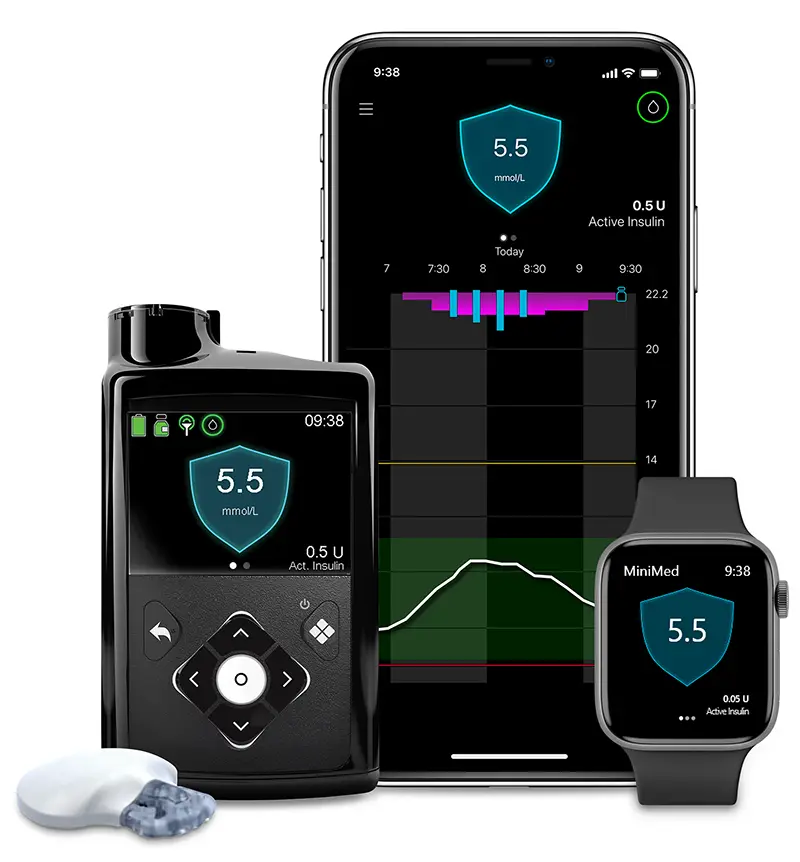
With MiniMed™ 780G, the only available system^ that automatically adjusts & corrects every 5 minutes, so you don’t have to.* 1,2
We are excited to introduce you to AccessPlus+, a revolutionary program from Medtronic designed to give you faster access to the latest in insulin pump technology.
Get started today Speak with us
^ Components sold separately
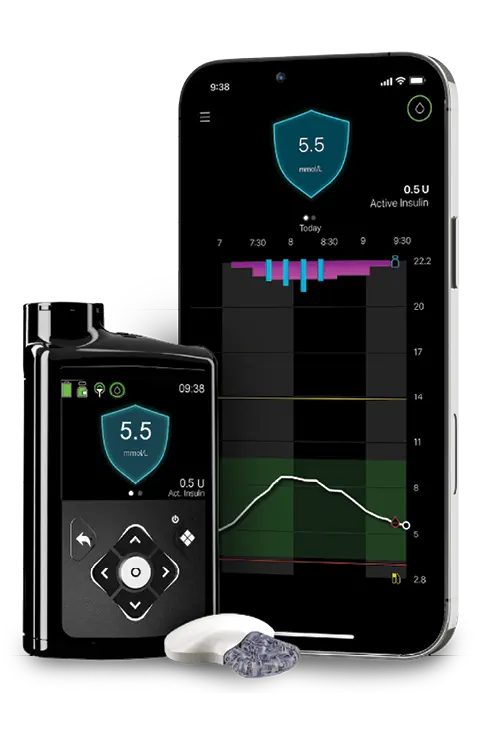
What is AccessPlus+?
AccessPlus+ allows you to start using a Medtronic insulin pump for up to 12 months, even if your current pump warranty is still active.
Benefits of AccessPlus+
Start Sooner
Begin using advanced insulin pump technology immediately.
Expert Support
Ongoing assistance from Medtronic and your healthcare team.

Who is Eligible?
To be eligible for AccessPlus, you need to meet the following criteria*:
- Type 1 Diabetes, Australian Resident, been prescribed an insulin pump by your Healthcare Professional (HCP).
- You are not currently using an in-warranty or loan Medtronic MiniMed™ 770G or MiniMed™ 780G insulin pump.
- You are eligible for funding of the Medtronic continuous glucose monitoring (CGM) through the National Diabetes Services Scheme (NDSS).
And one of the following
- You are currently using insulin pump technology including: mylife YpsoPump, OmniPod DASH, t:slim X2 or others, with a warranty date ending within 12 months
- You have private health insurance that covers insulin pumps or you can pay $AUD 8,574 for the pump.
How to Apply?
- Discuss your suitability for AccessPlus+ with your diabetes healthcare professional.
- Fill out the AccessPlus+ application form and send via online form, email or fax.
- Upon approval receive confirmation email from Medtronic.
- After approval, receive your new insulin pump along with necessary training and support.
Upgrade your diabetes management today with Medtronic’s AccessPlus+.
To learn more, fill out your details in the form below, or contact our team at 1800 777 808 and select option 3.
Apply now
Discover a Faster Path to the Latest Insulin Pump Technology
Don’t wait - AccessPlus lets you start using a Medtronic insulin pump earlier. Request a call today to see how you can begin your journey to better diabetes management with expert support from a Diabetes Therapy Consultant.
ALWAYS FOLLOW THE DIRECTIONS FOR USE.
^Components sold separately.
The MiniMed 780G insulin pump is indicated for use by patients age 7-80 years with Type 1 diabetes, whose total daily dose of insulin is 8 units per day or more. The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts. The system is also intended to continuously monitor glucose values in the fluid under the skin. The MiniMed 780G system includes SmartGuard technology, which can be programmed to provide an automatic adjustment of insulin delivery based on continuous glucose monitoring (CGM) and can suspend the delivery of insulin when the SG value falls below, or is predicted to fall below, predefined threshold values.
Automated insulin delivery is made possible through combining Medtronic insulin pump and continuous glucose monitoring technology. Some user interaction required. Individual responses to the treatment can and do vary Consult a healthcare professional to see if this product is suitable for you.
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG)readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
References
* If sensor glucose (SG) values do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions. Refer to System User Guide.
1. Carlson, A.L. et al. Safety and Glycemic Outcomes During the MiniMed Advanced Hybrid Closed-Loop System Pivotal Trial in Adolescents and Adults with Type 1 Diabetes. DIABETES TECHNOLOGY & THERAPEUTICS. Vol 24, No. 3 (2022) 1-12.
2. Da Silva J, et al. Real-world Performance of the MiniMed™ 780G System: First Report of Outcomes from 4'120 Users. Diabetes Technol Ther. 2021; doi: 10.1089/dia.2021.0203
Approval# AU-IPT-2400007