Stay ahead of diabetes without fingersticks*
Spend more time doing what you enjoy with a smart continuous glucose monitor (CGM) that helps you stay ahead of highs and lows, 24/7.
Guardian 4
smart CGM system
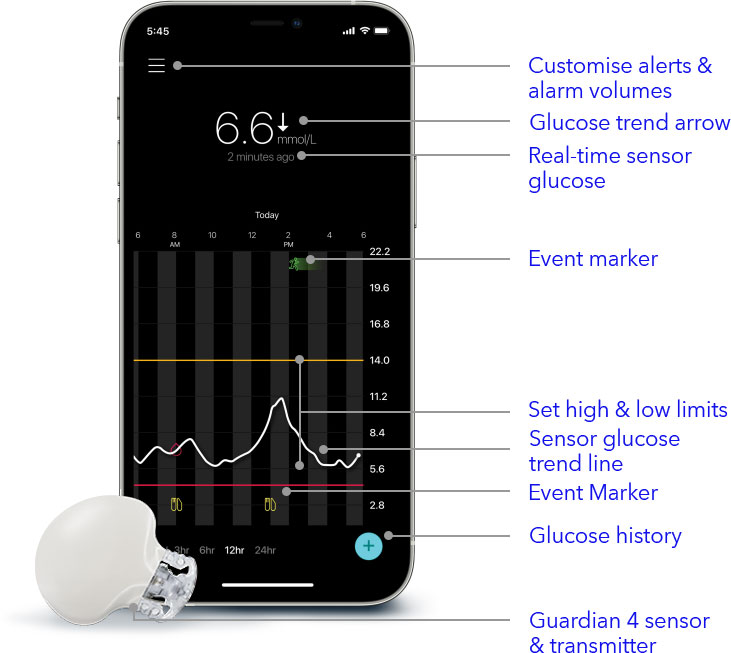
Guardian 4
smart CGM
Stay ahead with smarter technology
- Real-time glucose readings
See how you're trending on your mobile device with sensor glucose readings every 5 minutes. - Advanced predictive glucose insights
Know up to 1-hour in advance of a high or low 24/7. 1,† - Advanced notifications, alerts, and alarms
Set and customise your glucose notifications, alerts, and alarm volumes to meet your preferences. - Activity Diary
Record insulin, exercise, meals, and more in the integrated logbook to understand how activities may affect your glucose trends.

Stay connected with shareable data
Share glucose data with your friends and family.
With remote monitoring, you can keep those who are important to you informed about your data. Share
changes in your glucose values by having real-time text alerts sent securely to their mobile phone.
Share personalised glucose reports.
Discover new ways to improve your therapy by collaborating with your healthcare provider with personalised glucose management reports from CareLink™ Personal software.

Stay on track for improved health2
Get real results with the Guardian 4 system2
- Predictive alerts can help reduce hypoglycemia and help maintain time in range.3,4
- Predictive glucose alerts can help reduce hypoglycaemia (low) events by 59% and hyperglycaemia (high) events by 39%.5

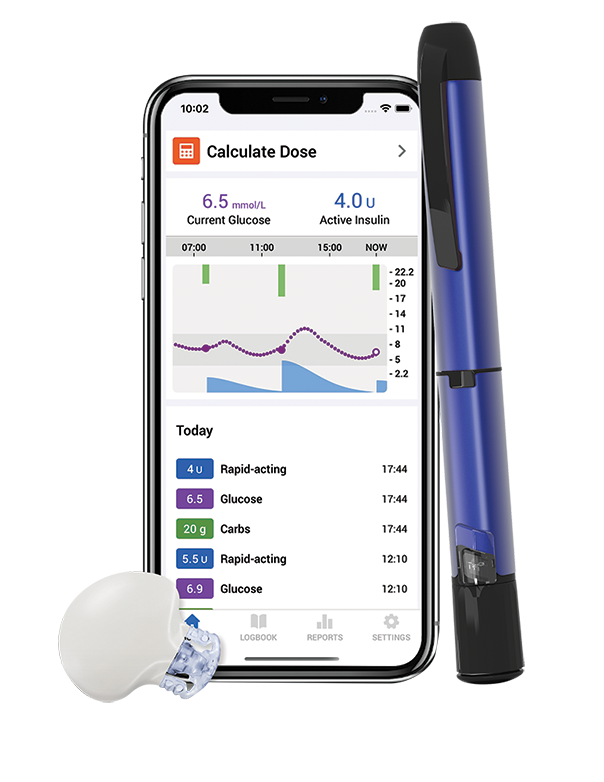
Smart MDI System
The Medtronic Smart MDI system includes InPen™ and Guardian™ 4 Sensor, with features that allow you to stay ahead of your glucose levels.
- Real time CGM with predictive glucose alerts
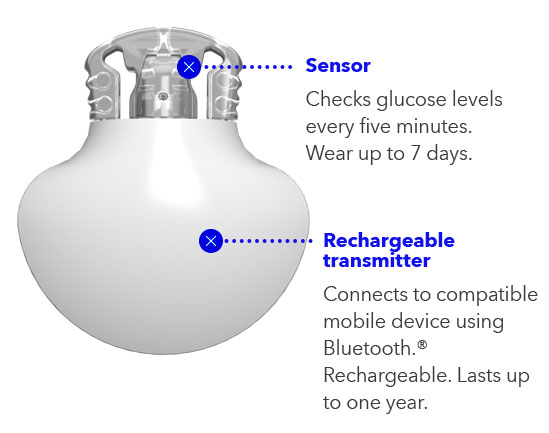
- Records glucose levels every 5 minutes, 24/7 with no fingersticks*
- Alerts of lows and highs up to 60 minutes ahead of time with no fingersticks*
- Personalised insulin dosing
- Insulin tracking
- Dose calculator
- Dose reminder
Learn more CGM and InPen apps features
Enter your information and we'll contact you with more information about the Guardian 4 smart CGM system.
Request a call today, to discuss the benefits of Guardian™ Connect with a Medtronic Diabetes Therapy Consultant.
References
® The Bluetooth(R) wordmark and logos are registered trademarks owned by Bluetooth SIG, Inc. and any use of such marks by Medtronic is under license.
* Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
§ vs. using BG monitoring, or a CGM system with no predictive alert capability
† Proper mobile device, settings and human interaction required.
- User interaction required. Diabetes treatment decisions should be made based on a combination of SG readings, trend arrows, glucose target ranges, active alerts, and recent events (such as insulin doses, exercise, meals, and medications)
- Miller, K., Kanapka, L., Bauza, C., Laffel, L. 898-P: Benefit of Continuous Glucose Monitoring (CGM) in Reducing Hemoglobin A1c Is Sustained through 12 Months of Use among Adolescents and Young Adults with Type 1 Diabetes (T1D). Diabetes. 2020;69(Supplement 1). doi:10.2337/db20-898-p
- Haskova A, et al. Real-time CGM Is Superior to Flash Glucose Monitoring for Glucose Control in Type 1 Diabetes: The CORRIDA Randomized Controlled Trial. Diabetes Care 2020;43:2744-2750
- Battelino T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range Diabetes Care 2019;42:1593-1603
- Abraham SB, et al. Improved Real-World Glycemic Control with Continuous Glucose Monitoring System Predictive Alerts. Journal of Diabetes Science and Technology 2021; 15(1):91-97
ALWAYS FOLLOW THE DIRECTIONS FOR USE
A healthcare professional should assist in programming of the device prior to use, based on various patient-specific criteria and targets. For additional product and safety information, please consult the Instructions for Use.
InPen™ is intended for single-patient use by people with diabetes for the self-injection of a desired dose of insulin.
The Guardian 4 sensor is intended for use with the Guardian 4 transmitter to monitor glucose levels in persons with diabetes where self-monitoring of blood glucose (SMBG) is indicated. The sensor is designed to replace fingerstick blood glucose (BG)readings for diabetes treatment decisions. The sensor is intended for insertion into persons ages 7 years and older. The sensor is intended for insertion into the back of the upper arm or the upper buttocks in persons ages 7 through 17 years. The sensor is intended for insertion into the back of the upper arm or the abdomen in persons ages 18 years and older.
©2023 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Further, Together are trademarks of Medtronic. SpringCM Approval# 13245-092023. Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. Android is a trademark of Google LLC
